On December 8, 2023, the Beijing Municipal Science & Technology Commission, Administrative Commission of Zhongguancun Science Park announced the second batch of the 2023 (totaling the 19th batch) list of recognized "Beijing New Technology, New Product, and New Service." Two products from AccuMedical Beijing Ltd., including the Flow Diverter and Distal Access Catheter, have successfully passed the certification as "Beijing New Technology & Product (Service)".
Image: Announcement of the second batch of 2023 (totaling the 19th batch) of Beijing New Technology, New Product, and New Service certification.
The Beijing New Technology & Product (Service) recognition is a highly authoritative and influential recognition program. It selects advanced technological products and services with clear intellectual property rights, reliable quality, and broad market prospects from key leading technologies, strategic emerging industries, and modern service sectors, aligning with Beijing’s "High-End, Precision, and Cutting-Edge" economic structure. Recognized new technology and products (services) can enjoy government procurement and promotion support and be included in the national procurement platform for new technologies, products, and services.
This recognition aims to promote the development of high-tech industries and the application of new technologies and products (services), providing momentum for enterprise innovation and research capability enhancement. Being selected this time is a strong endorsement by five departments, including the Beijing Science and Technology Commission and the Beijing Development and Reform Commission, of AccuMedical’s innovation and leadership in the field of neurointervention medical devices. The two successfully recognized products focus on the treatment of hemorrhagic cerebrovascular diseases and innovative solutions, addressing urgent clinical needs and already being applied in practice.
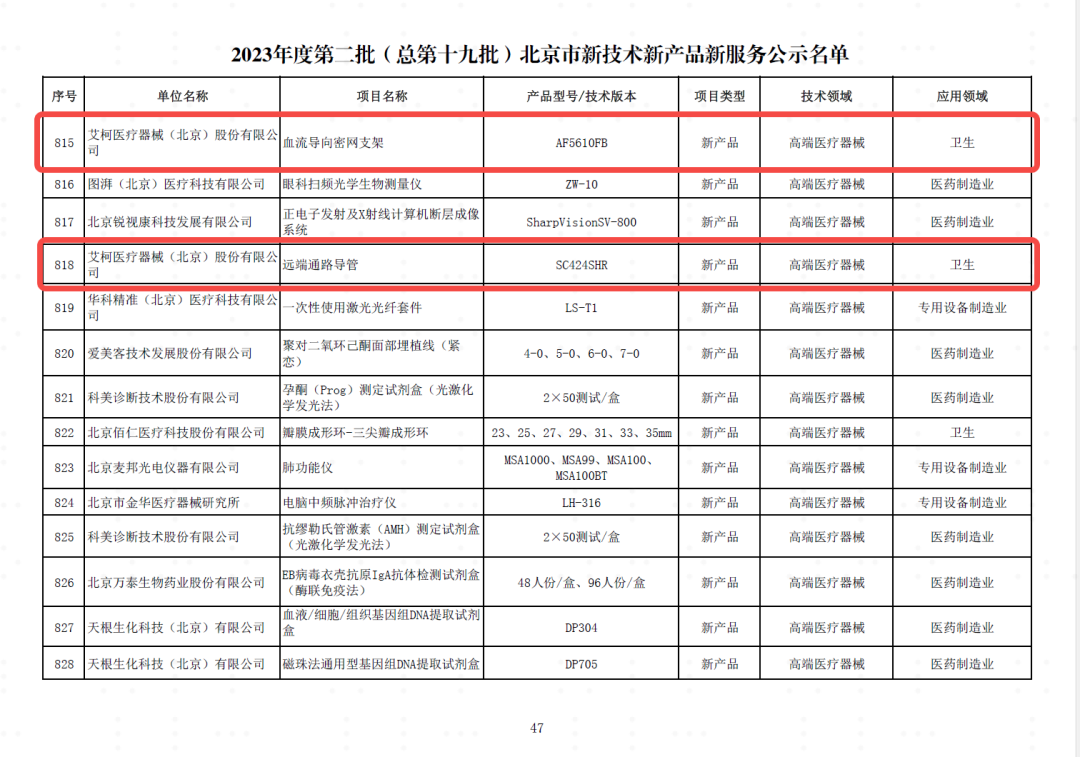
Image: AccuMedical selected in the second batch of 2023 (totaling the 19th batch) of Beijing New Technology, New Product, and New Service certification list.
Founded in 2017, AccuMedical Beijing Ltd. is an innovative medical device company specializing in the field of neurointervention. Headquartered in Beijing with dual R&D centers in China and the United States, AccuMedical has developed comprehensive businesses including R&D, production, and marketing of neurointerventional devices for the treatment of hemorrhagic and ischemic strokes. Our mission is to offer a whole set of innovative solutions to a global population of cerebrovascular disease patients and physicians. Since its inception, AccuMedical has been dedicated to breakthroughs and advancements in key technologies and processes, focusing on high-barrier, high-clinical-value neurointervention products. As of December 2023, the company has filed over 88 patents, with 43 already granted, including multiple world-first technologies.
The interventional treatment of intracranial aneurysms has entered the era of Flow Diverters (FD). Unlike traditional coil embolization within an aneurysm sac, FDs work by altering blood flow within the parent artery and reconstructing the vascular structure. This reduces the direct impact of blood flow on the aneurysm, leading to blood stagnation and thrombus formation within the aneurysm sac. Moreover, new endothelial cells grow over the surface of the FD at the aneurysm neck, eventually achieving complete aneurysm occlusion while reducing the risk of recurrence. According to the "Chinese Guidelines for Flow Diverter Treatment of Intracranial Aneurysms", FD treatment is highly effective and safe for unruptured wide-neck aneurysms in the internal carotid artery, including small, medium, large, and giant aneurysms. It is rated as a Class I recommendation with Level B evidence. AccuMedical’s Lattice Flow Diverter is currently the most widely approved FD in China, covering both anterior and posterior circulations. It is indicated for treating large/giant, medium, and small aneurysms, as well as saccular and fusiform aneurysms. This extensive applicability allows AccuMedical to offer broader treatment options for patients and address a wider range of cerebrovascular conditions.